Background. Before the recent introduction of daratumumab (Dara) in the frontline setting, lenalidomide-dexamethasone (Rd) has represented a standard of care for transplant-ineligible (NTE), patients (pts) with newly diagnosed multiple myeloma (NDMM). Nonetheless, minimal residual disease negativity (MRD Neg) rate and median progression-free survival (PFS) with Rd were still relatively limited, as compared with 3/4-drug regimens, also including carfilzomib (K). The EMN20 trial (NCT04096066) is a randomized, multicenter study designed to compare weekly K in addition to Rd (KRd) with Rd in fit or intermediate-fit NTE NDMM pts, with the objective of attaining improved rates of PFS and 2-year MRD Neg through a triplet regimen.
Methods. Fit or intermediate-fit NTE NDMM pts (according to the International Myeloma Working Group frailty score) were randomly assigned to receive KRd (28-day cycles, once-weekly K 56 mg/m² on days [dd] 1,8,15 for 12 cycles and on dd 1,15 from cycle 13 onwards for 5 years (yr), R 25 mg orally on dd 1-21 continuously and d 40 mg on dd 1,8,15,22 continuously) or continuous Rd (28-day cycles, R 25 mg on dd 1-21, d 40 mg on dd 1,8,15,22). Pts were stratified based on International Staging System (ISS) stage and fitness status, using a web-based procedure completely concealed from study participants. Patients in the KRd arm sustaining MRD Neg after 2 yr of treatment will discontinue carfilzomib early (at 2 yr) and continue Rd alone. The primary endpoints were MRD Neg after 2 yr of treatment and PFS. For MRD assessment, the clonoSEQ™ assay was used at the sensitivity of ≥10⁻⁵. The MRD Neg rate was the proportion of MRD-Neg pts (sensitivity ≥10⁻⁵) at 2 treatment yr. Key secondary endpoints included response rates, overall survival (OS) and safety. The protocol was prematurely stopped on Nov 23, 2021, after the introduction of frontline Dara-Rd.
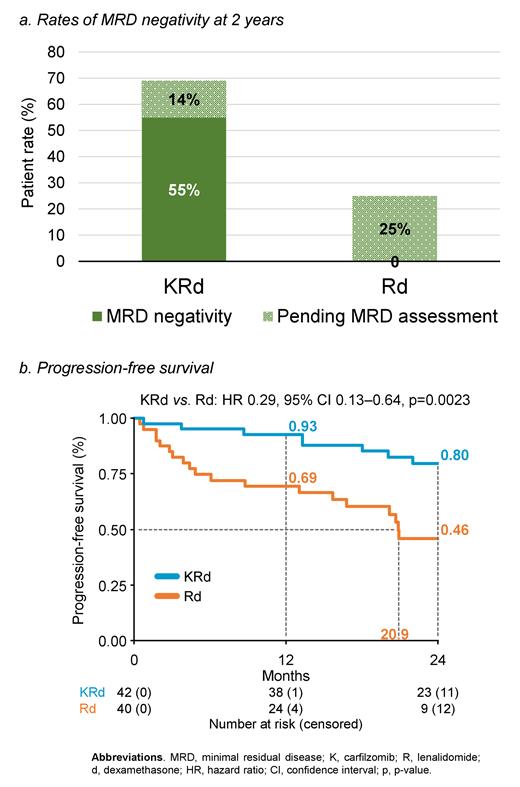
Results. A total of 101 pts were enrolled and 82 were randomized (KRd 42 vs Rd 40); 19 pts were not randomized due to screening failure (17) and withdrawal of consent (2). Pt characteristics were well balanced between the KRd and Rd arms: median age was 73 (IQR 70-76) and 74 yr (IQR 72-76), 60% vs 58% of pts were fit, 33% vs 30% had ISS III and 22% vs 22% had high-risk cytogenetics, respectively. In the KRd vs Rd arms, 33/42 (78.6%) vs 18/40 (45.0%) pts are still under treatment; reasons for discontinuation were medical decision (1 vs 4), death (2 vs 5), adverse events (AEs; 2 vs 1), progressive disease (3 vs 10), lost to follow-up (1 vs 0) and consent withdrawal (0 vs 2). After a median follow-up of 24.9 months (IQR 20-29), 79% of KRd pts and 50% of Rd pts were alive and progression-free. In the ITT population, MRD Neg rates (at 10⁻⁵) were observed in 21/42 (50%) KRd pts vs. 0 Rd pts at 1 yr of treatment (p<0.0001) and in 23/42 (55%) KRd pts and 0 Rd pts at 2 yr (p<0.0001). 16/42 (38%) KRd pts and 0 Rd pts sustained MRD Neg at 2 yr (p<0.0001; Figure panel a). At the time of abstract submission, the assessment of MRD at 2 yr of treatment was still pending for 6 KRd pts and 10 Rd pts. Median PFS was not reached with KRd vs 20.9 months with Rd (HR 0.29, 95% CI 0.13-0.64, p=0.002; Figure panel b). In multivariable analysis, no significant effect modification was observed in terms of ISS or frailty status. 2-yr OS was 89% with KRd vs 74% with Rd (HR 0.36, 95% CI 0.11-1.17, p=0.09). The most frequent grade 3-4 AEs with KRd were neutropenia (22%), thrombocytopenia (10%), cardiac AEs (7%), infection (7%) and hypertension (5%), while with Rd they were neutropenia (12%) and dermatologic AEs (10%). Second primary malignancy was observed in 1 pt (3%) in the Rd arm. Eighty-five percent of pts in the KRd and 70% in the Rd arms had ≥1 dose reductions of any drug.
Conclusions. To our knowledge, these results demonstrate for the first time an unexpected high rate of MRD Neg in NTE NDMM pts. Half of pts treated with KRd achieved MRD Neg, improving over time with therapy (50% at 1 yr and 55% at 2 yr), and 38% of KRd patients sustained MRD Neg at 2 yr. This higher rate of MRD Neg was associated with prolonged PFS. Toxicities were predictable and manageable. Updated results will be presented at the meeting.
OffLabel Disclosure:
Bringhen:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Honoraria; Sanofi: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees. Gamberi:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Fazio:Amgen: Honoraria; Takeda: Honoraria; Janssen-Cilag: Honoraria. Corradini:Incyte: Other: Honoraria (Consulting, advisory role, or lecturer); Daiichi Sankyo: Other: Honoraria (Consulting, advisory role, or lecturer); Kyowa Kirin: Other: Honoraria (Consulting, advisory role, or lecturer); Takeda: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; SOBI: Other: Honoraria (Consulting, advisory role, or lecturer); Sanofi: Other: Honoraria (Consulting, advisory role, or lecturer); Roche: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Pfizer: Other: Honoraria (Consulting, advisory role, or lecturer); Novartis: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Janssen: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Nerviano Medical Science: Other: Honoraria (Consulting, advisory role, or lecturer); Gilead/Kite: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Celgene: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Amgen: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; GlaxoSmithKline: Other: Honoraria (Consulting, advisory role, or lecturer); AbbVie: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; ADC Theraputics (DSMB): Other: Honoraria (Consulting, advisory role, or lecturer); BeiGene: Honoraria; Bristol Myers Squibb: Other: Travel and accomodations. Belotti:Takeda: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees. Larocca:Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees. Boccadoro:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; AbbVie: Honoraria; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Mundipharma: Research Funding; Amgen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding. D'Agostino:Sanofi: Membership on an entity's Board of Directors or advisory committees, Other: Honoraria for lectures; Janssen: Other: Honoraria for lectures; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees, Other: Honoraria for lectures.
This presentation includes information or discussion of the off-label use of a drug or drugs for the treatment of multiple myeloma: carfilzomib, lenalidomide, and dexamethasone.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal